Klinische Studien
Grifols' Engagement in der Alzheimer-Forschung begann vor fast 20 Jahren, als das Unternehmen beschloss, das Potenzial von Plasmatherapien zur Behandlung dieser verheerenden neurodegenerativen Krankheit zu untersuchen. Die präklinischen Versuche begannen 2004 mit zwei präklinischen Pilotstudien und einer klinischen Studie der Phase 2, bevor die AMBAR®-Studie gestartet wurde.
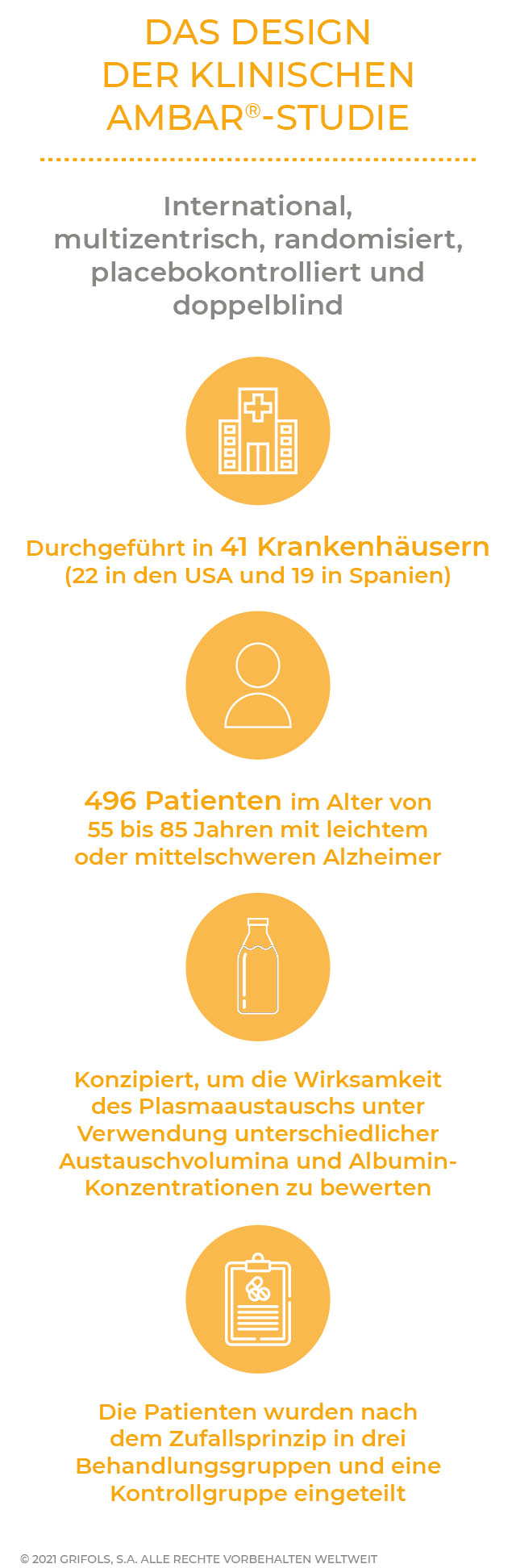
Bei der klinischen AMBAR®-Studie handelte es sich um eine internationale, multizentrische, randomisierte, doppelblinde, parallel angelegte und placebokontrollierte Studie, an der Patienten mit leichter und mittelschwerer Alzheimer-Erkrankung aus 41 Behandlungszentren in Spanien und den Vereinigten Staaten teilnahmen.
Es sollte untersucht werden, ob das Fortschreiten der Alzheimer-Krankheit durch TPE stabilisiert werden kann, ein Verfahren, bei dem in regelmäßigen Abständen Plasma entnommen und eine Albuminlösung infundiert wird.
Die Studie, die von Grifols in Zusammenarbeit mit dem Ace Alzheimer Center Barcelona und dem Alzheimer's Disease Research Center in Pittsburgh (USA) konzipiert wurde, hatte zum Ziel, die Wirksamkeit und Sicherheit des Plasmaaustauschs mit Albumin (in einigen Behandlungsarmen in Kombination mit intravenösem Immunglobulin) bei Patienten mit leichter und mittelschwerer Alzheimer-Ekrankung zu untersuchen.
Die ermutigenden Ergebnisse der klinischen Studie wurden in zwei Artikeln in der renommierten Zeitschrift Alzheimer's & Dementia: The Journal of the Alzheimer's Association veröffentlicht und auf mehreren wissenschaftlichen Konferenzen, die sich mit der Alzheimer-Erkrankung befassen, vorgestellt, darunter Clinical Trials on Alzheimer's Diseases (CTAD), International Conference on Alzheimer's and Parkinson's Diseases (AD/PD) und Alzheimer's Association International Conference (AAIC).
Im Mai 2021 eröffnete das Grifols Ace Alzheimer Center Barcelona das erste AMBAR® Center, in dem das AMBAR® Verfahren in einer realen Umgebung angewendet wird.

Grifols' innovative Alzheimer-Forschung
Von der Entdeckung bis hin zu klinischen Entwicklungsprogrammen machen wir in unserem Kampf gegen diese Krankheit weiterhin Fortschritte.