Bags for parenteral solutions
New materials and technological innovation
Technical file
Type of innovation: Device; System; Procedure
Scope: Hospital Pharmacy
Innovation leader: Grifols i Lucas, Víctor
Year: 1989
Period: 1972-2002
Geographical scope: International
Economic impact: High
Level of innovation: Disruptive
Patent: Yes
Interdisciplinary connections: -
Grifols has been manufacturing parenteral solutions for the hospital pharmacy sector since the 1950s. These medicines require the very highest levels of safety as they are injected directly into the bloodstream, without any biological barrier. This means that extremely strict processes must be followed when handling them and, more generally, to ensure the absence of bacteria, viruses and other microorganisms.
In addition to manufacturing the parenteral solutions themselves, Grifols also had a track record of developing containers and improving the process of filling and sterilizing these medicines, responding rapidly to the appearance of technological innovation in the sector, and working to improve the safety and reliability of its products.
From glass to plastic
Parenteral solutions for hospital pharmacy were traditionally bottled in glass containers, a material that was chosen because it was neutral, and was suitable for the transport and conservation of medicines for intravenous infusion. However, in the mid-1980s a number of studies were performed to identify a more flexible alternative to the traditional glass containers, and these concluded that PVC was the best material for conserving such solutions. PVC was also favored by nursing staff, who preferred it because it eliminated the problem of breakages.
In response to this market trend, Grifols decided to introduce flexible packaging for its fluid therapy products. However, a number of problems first had to be overcome, both to guarantee the safety and practicality of the new containers, and to adapt the filling process to the new material.

The double bag
The initial assumption that plastic was impermeable turned out to be incorrect: over time, some of the liquid inside plastic containers evaporates. This led to problems when hospitals had to infuse a specific quantity of solution and found that the volume of solution in the container was actually less than they required. Most companies responded to this by enclosing the intravenous solution bag within a second bag to prevent evaporation. The double bag was designed to ensure greater protection and safety in two ways: the risk of contamination as a result of handling was reduced, and the second bag prevented the liquid inside from evaporating.
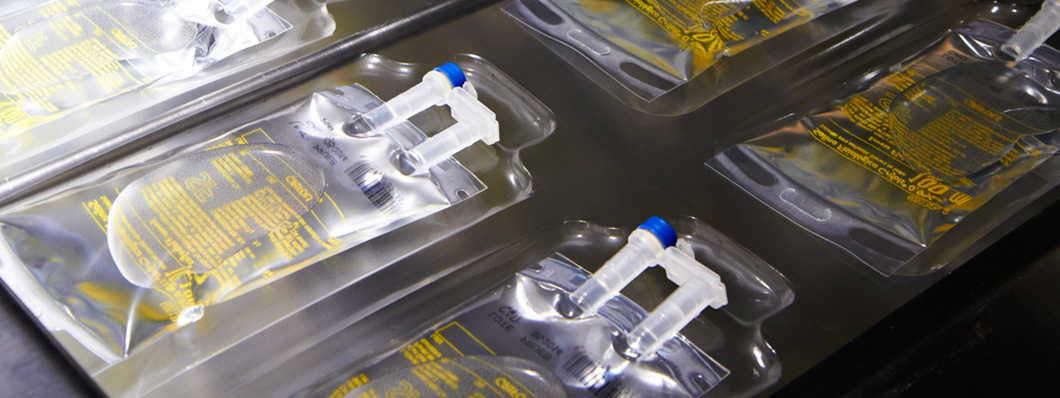
“The shift from glass to plastic meant that the industrial processes for filling and sterilizing parenteral solutions had to be modified. Víctor Grifols i Lucas, technical director of Grifols at the time, solved the problem by designing a machine that was patented in 1990.”
A new filling system
The shift from glass to plastic meant that the industrial processes for filling and sterilizing parenteral solutions had to be modified. Víctor Grifols i Lucas, technical director of Grifols at the time, solved the problem by designing a machine that was patented in 1990. In the new procedure, the empty bag was placed inside a mold, and a vacuum applied. Next, the bag was connected to a tank containing the solution, which was dispensed at atmospheric pressure. The inlet was then sealed once the bag was full. This system meant that the solution entered the bag directly, without the requirement for any kind of valve or joint that might carry the risk of particle contamination.

Using internal pressure to ensure sterility
The only way to ensure that a solution is free of pyrogens is to manufacture it under completely sterile conditions. This typically involves the use of autoclaves in which the solution is subjected to thermal treatment under pressure for a number of hours. The shift from glass to plastic created a problem because some bags tore as a result of the sharp drop in pressure and temperature during the cooling and decompression stage. Grifols solved this problem by studying the internal pressures applied in the autoclave and applying far more gradual counter-pressures. The bags were thus subject to less stress during the cooling phase.
Integrated injection and infusion ports
Another innovation introduced by Grifols took the form of an integrated injection and infusion port. Traditionally, these ports had been separate, incorporating a number of components. Grifols integrated injection and infusion port offered a number of advantages, both in terms of the manufacturing process and with regard to ergonomics and safety. The integrated port was easier to hold and was more rigid, reducing the risk of needlestick injuries. Moreover, because there were no snap-off disposable parts, the new design was also more environmentally friendly.

Plastic containers for parenteral solutions
Flebobag®: a flexible PVC bag from Grifols
Flebobag® was Grifols first plastic container for parenteral solutions, and it was so popular that Grifols needed two suppliers of PVC bags to cover demand for the product. By 2003, the company was producing 14 million bags of intravenous solutions per year, destined primarily for the Spanish hospital network.
Fleboflex®: polypropylene bags and automated manufacturing
In 2005, Grifols launched the Fleboflex® bag, manufactured from polypropylene. Growing demand for the product in the years that followed led to the automation of the manufacturing lines in 2009, and the construction of a new plant for parenteral solutions in a flexible polypropylene container. With the new facility, capacity increased by 30 million bags per year, to a total of 44 million.
Bibliography
Grifols i Lucas, V. (1960). Perfeccionamiento en los medios de control automático de la presión y tiempo de esterilización en autoclave. (Oficina Española de Patentes y Marcas Patente Nº 261354). Link
Grifols i Lucas, V. (1966). Bolsa para la transfusión de soluciones gota a gota dotada de racord de alimentación con cierre estanco que se abre desde el exterior. (Oficina Española de Patentes y Marcas Patente Nº 120126). Link
Grifols i Lucas, V. (1989). Procedimiento perfeccionado para el llenado de bolsas con líquidos a perfundir e instalación para la puesta en práctica de dicho procedimiento. (Oficina Española de Patentes y Marcas Patente Nº ES2016489 (A6)). Link
Grifols i Lucas, V. (1989). Procedimiento perfeccionado para recubrir una bolsa de plástico que contiene líquido para perfusión con otra bolsa, máquina para la puesta en práctica de dicho procedimiento y bolsa obtenida en el mismo. (Oficina Española de Patentes y Marcas Patente Nº ES2016492 (A6)). Link
Roura Fernández, C. y Menéndez Cortina, J. (2005). Bolsa para soluciones terapéuticas. (Oficina Española de Patentes y Marcas Patente Nº 200501664). Link
Grifols, S.A. (2011). ¿Y si lo hacemos así? Patentes de Víctor Grífols i Lucas. Barcelona: Grifols, S.A.
Roura Fernández, C., Garcia Garcia, J.A., Llorens Masas, E. y Marzo Adam, N. (2016). Double bag. Contenedor para una solución de proteínas plasmáticas humanas y procedimiento de preparación del mismo. (Oficina Española de Patentes y Marcas Patente Nº 201631198). Link
Related innovations
FLUID THERAPY
Intravenous solutions