The Gri-Fill® filling system
A closed system for the sterilized filling of intravenous mixture bags
Technical file
Type of innovation: Instrument; Procedure; System
Scope: Hospital Pharmacy
Innovation leader: Grifols i Lucas, Víctor
Year: 1993
Period: 1972-2002
Geographical scope: International
Economic impact: High
Level of innovation: Disruptive
Patent: Yes
Interdisciplinary connections: -
By the late 1960s, hospitals were beginning to establish dedicated units to handle the preparation and distribution of intravenous mixtures. Because intravenous medicines are introduced directly into the patient's bloodstream, they have to be prepared with great care, under sterile conditions.
Grifols began manufacturing parenteral solutions for hospital pharmacy services in the 1950s, and this meant the company's staff were in constant communication with health professionals. As a result, Grifols was well placed to identify the challenge posed by the need to prepare intravenous mixtures with the doses of individual patients, and to develop a solution to this problem.
Filter control
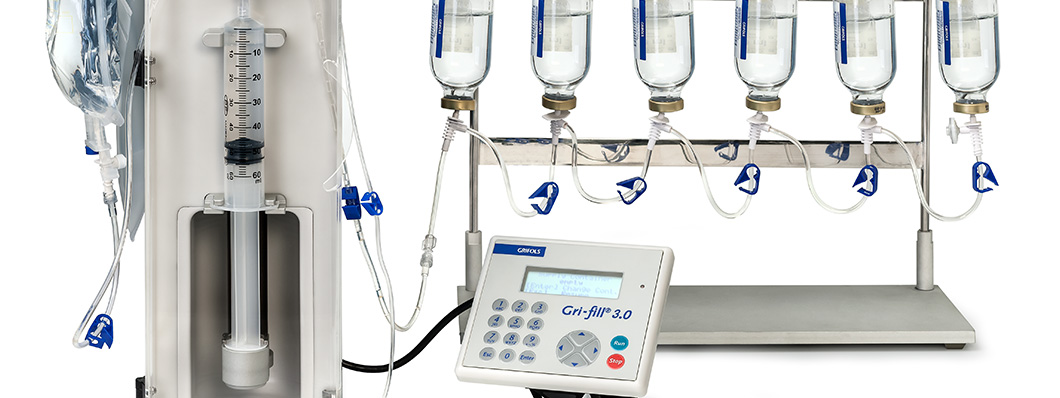
In the late 1980s, Grifols worked with the Hospital Marqués de Valdecilla in Santander in northern Spain, to develop a closed system for filling small-volume bags with ranitidine solution, which could not be sterilized in an autoclave, the usual method for sterilizing mixtures at the time. The system designed by Víctor Grifols i Lucas combined a PVC bag with a filling tube that incorporated a filter, which made it possible to control the sterility of the solution. In addition, the team developed a machine that was capable of filling six bags at once, and also automatically checked the integrity of the filter.
“The benefits of the new system included fewer preparation errors, lower incidence of nosocomial disease, greater traceability, and better care, as doses more closely reflected patient needs.”
Combining pump, tubes and bag
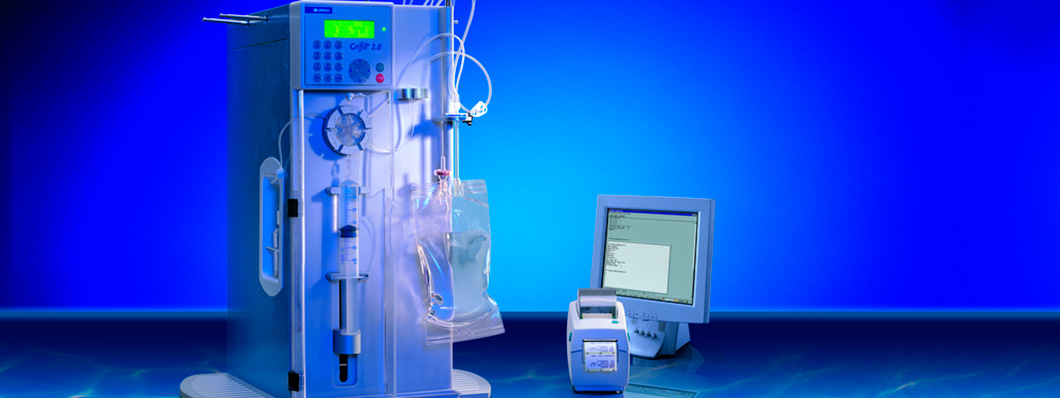
The next stage was to develop the system for use in hospital pharmacies to prepare intravenous mixtures that could be customized to meet the needs of individual patients who required formulas in non-standard volumes, contents and proportions, which were not viable to manufacture at an industrial scale. Grifols solution consisted of a closed filling system – Gri-Fill® – composed of a peristaltic pump, a set of tubes and, the most innovative feature of all, a bag – the Gri-Bag® – which incorporated a sterilizing filter.

In-built quality control
To ensure sterility, quality control was performed on each unit, using the bubble point technique. This involves subjecting the filter to air pressure to check the integrity of the filter, once the bag has been filled. If the filter is intact, it prevents any particle larger than 0.22 microns from passing through. (The smallest bacteria measure 0.3 microns in diameter.) This method meant that dosing did not need to be performed in sterile rooms, and the Gri-Fill® system could thus be used in any hospital pharmacy.
The first unit of the system was installed in the pharmacy service of the Hospital Marqués de Valdecilla, and the Gri-Fill® system was launched commercially in 1993, offering hospitals on-site preparation of sterile intravenous mixtures of pharmaceutical products with individualized doses adjusted to the needs of patients. The benefits of the new system included fewer preparation errors, lower incidence of nosocomial disease, greater traceability, and better care, as doses more closely reflected patient needs.

An evolving system
The next generation of the system, Gri-Fill® 2.0, was presented in 2002 and incorporated several improvements, including the replacement of the peristaltic pump with a syringe pump and disposable kit, which dispensed with the need to clean and sterilize the whole circuit after each use.
This was followed by the Gri-Fill® 3.0, which was small enough to be used inside a laminar flow cabinet with negative pressure, and could thus be used to prepare chemotherapy drugs. It was validated by the University of California in 2005 and released in 2006.
Gri-Fill® 4.0 was launched in 2020. This incorporates two main innovations. Its software enables it to work on line with hospital prescription systems. And it has a double pump: peristaltic for large volumes of ‘mother' solution and volumetric for high-precision dosing of medications.
Bibliography
Grifols i Lucas, V. (1991). Bolsa para líquidos de perfusión perfeccionada. (Oficina Española de Patentes y Marcas Patente Nº ES2110457 (T3)). Link
Grifols i Lucas, V. (1993). Dispositivo para la conexión múltiple de frascos para infusiones médicas. (Oficina Española de Patentes y Marcas Patente Nº ES1025054). Link
Grifols i Lucas, V. (1993). Máquina para el llenado estéril y su comprobación de bolsas estériles para líquidos de perfusión. (Oficina Española de Patentes y Marcas Patente NºES1024044). Link
Grupo Grifols. (1995). Dosificadora GRI-FILL. Un proyecto 100% Grifols. Revista Cosmos. Periódico para los colaboradores del Grupo Grifols, 2(1), 5.
Menéndez, M. (1997). Guía para el desarrollo de servicios farmacéuticos hospitalarios: preparación de mezclas de uso intravenoso. Buenos Aires: Organización Panamericana de la Salud, Organización Mundial de la Salud.
Grifols i Lucas, V. (2001). Aparato para el llenado de recipientes para usos farmacéuticos y similares. (Oficina Española de Patentes y Marcas Patente Nº 200100473). Link
Grifols i Lucas, V. (2002). Aparato para el llenado de recipientes para usos farmacéuticos y similares. (Oficina Española de Patentes y Marcas Patente Nº ES2255772 (A1)). Link
Grifols, S.A. (2011). ¿Y si lo hacemos así? Patentes de Víctor Grífols i Lucas. Barcelona: Grifols, S.A.
Avellà, R., & Miquel, B. (Eds.). (2015). Cuando un sueño se cumple. Crónica ilustrada de 75 años de Grifols. Barcelona: Grupo Grifols, S.A
Related innovations
SYSTEM
The KIRO® robot-calibration needle