Production of Soyacal® and Tauramin®
The industrial production of lipid emulsions
Technical file
Type of innovation: Procedure
Scope: Hospital Pharmacy
Innovation leader: Grifols i Lucas, Víctor
Year: 1998
Period: 1972-2002
Geographical scope: Spain
Economic impact: Low
Level of innovation: Adaptive
Patent: Yes
Interdisciplinary connections: -
Clinical nutrition
Some patients require specific nutritional treatment because they find it difficult or impossible to take in food by regular means. This treatment is termed clinical nutrition and includes both enteral nutrition, which is administered via the gastrointestinal tract or orally, and parenteral nutrition, which consists of administering nutrients intravenously to patients admitted to hospital.
Parenteral nutrition at Grifols
As part of its product diversification policy, the Grifols group developed a clinical nutrition line that included both enteral and parenteral products, with Laboratorios Grifols manufacturing and marketing parenteral nutrition products such as Soyacal® and Tauramin®.
The truly innovative aspect of this project was not the specific products but rather the need to adapt a plant to produce these lipid emulsions. This was a very complex challenge, and one that could not be met by existing facilities for the manufacture of sterile solutions because the technology involved was quite different.
“The real innovation was the company's ability to successfully negotiate the complex process of adapting its manufacturing facilities to produce these lipid emulsions.”
Oxygen removal
Lipid solutions consist of drops of different sizes, and oxygen has to be removed from the solution because otherwise bubbles form. This requires procedures to be applied to extract all the oxygen from the equipment used to prepare the solutions. This was achieved by creating a machine called a ‘homogenizer', which applied counter-pressure and introduced nitrogen to extract all the oxygen present.
Rapid sterilization
Another challenge faced in the process of manufacturing these lipid solutions was that they could not be sterilized in the autoclave at 120 °C for 6 hours like non-lipid solutions, as they were unable to withstand more than 8 minutes of the heat treatment and pressure that such sterilization required. The solution to this problem came in the form of the revolving autoclaves used by Japanese firm Green Cross Corporation, a partner of Grifols in the 1980s. By fully rotating the autoclave, the solution could be heated far more quickly and completely homogenously, and this made it possible to complete the process of sterilizing the lipid solutions without destroying their properties. Grifols adapted this solution by designing revolving carriages for use inside standard autoclaves. The trays holding the solutions rotated while being subjected to pressure and heat, obtaining the same effect.
Soyacal®
The group launched Soyacal® lipid emulsion in 2000. Manufactured under license from the Japanese company Green Cross Corporation, Grifols staff traveled to Japan to learn about the production method with a view to adapting it to a more stable process. A few years later, the composition of the formula and the method of producing it would be modified to improve output and obtain a more resilient product.
In addition, the company moved to secure marketing authorizations for Italy, the United Kingdom, Portugal, Chile and Argentina, where Grifols had well-established subsidiaries. This resulted in overseas sales starting up in 2001, providing the springboard for the internationalization of the recently created line of parenteral nutrition products.

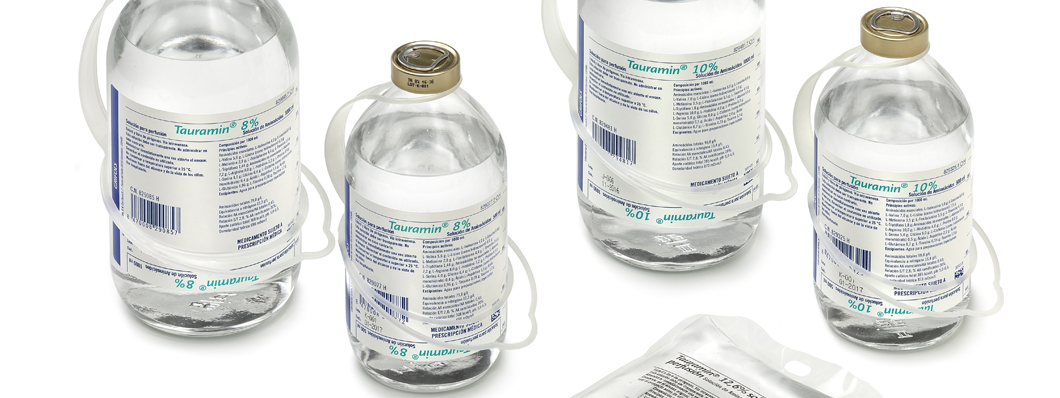
Tauramin®
The line was extended in 2005 with the launch of the amino acid solution Tauramin®, a product that broadened the range of nutrients administered intravenously to hospital patients and which was made up of other solutions of lipids and carbohydrates. The formula of the new product incorporated an innovation in the form of a concentration of taurine, which acts as a liver protector.
These formulas were extended in 2006 when the Spanish Agency of Medicines and Medical Devices (AEMPS) approved the new 8 and 10 percent solutions of Tauramin®, along with registration of a 10 and 20 percent lipid emulsion in Spain, Italy, the United Kingdom, and Germany. In the early 2010s, Grifols changed the packaging of Soyacal® and Tauramin®, replacing the original glass containers with new flexible bags made from PVC.
Bibliography
Grupo Grifols. (1999). Laboratorios Grifols amplía su oferta en nutrición parenteral. Revista Cosmos. Periódico para los colaboradores del Grupo Grifols, 12(2), 11.
Grupo Grifols. (2004). Grifols potencia la línea de Nutrición Clínica. Revista Cosmos. Periódico para los colaboradores del Grupo Grifols, 30, 8.
Avellà, R., & Miquel, B. (Eds.). (2015). Cuando un sueño se cumple. Crónica ilustrada de 75 años de Grifols. Barcelona: Grupo Grifols, S.A.